Test for CD123: A key marker due to its high expression (~95%) on BPDCN cells2-4
CD123, as part of a signature marker triad with CD4 and CD56, is a key marker in identifying BPDCN—a disease that can be challenging to distinguish from other hematologic malignancies.1,2


Key features of CD123 expression
- Highly expressed (~95%) on BPDCN cells and negligibly expressed on healthy cells2-5
- Can be both a diagnostic marker and a therapeutic target in BPDCN5
*BPDCN diagnosis can include other markers, such as TCL1, TCF4, and CD303 (BDCA-2).1,6
BPDCN is a pathologic diagnosis based on immunophenotype analysis through immunohistochemistry or flow cytometry1
Diagnosis of BPDCN requires multiple positive and negative markers1†
| Antigens that confirm the diagnosis of BPDCN1,6 | Antigens that exclude the diagnosis of BPDCN2,3,7-11 | Other markers that may be positive in BPDCN7,8 |
|---|---|---|
|
|
|
†One or more of these markers may be negative in some cases of BPDCN. Negativity does not rule out the diagnosis but does make it less likely.1,8
Recognizing BPDCN: Morphology
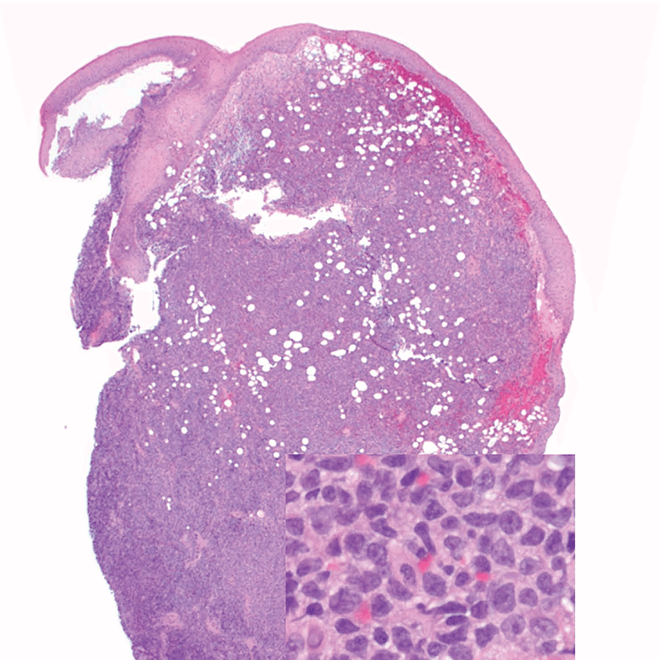
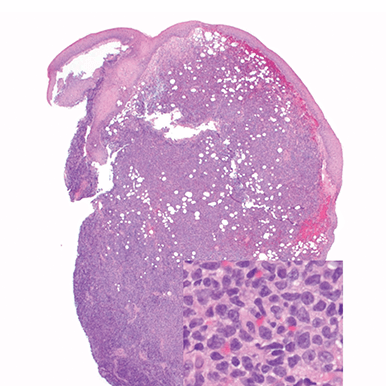
- Skin
- Punch biopsy of a skin lesion showing BPDCN (H&E stain, x40) and (inset) medium-sized malignant cells spare the epidermis (H&E stain, x1000).11
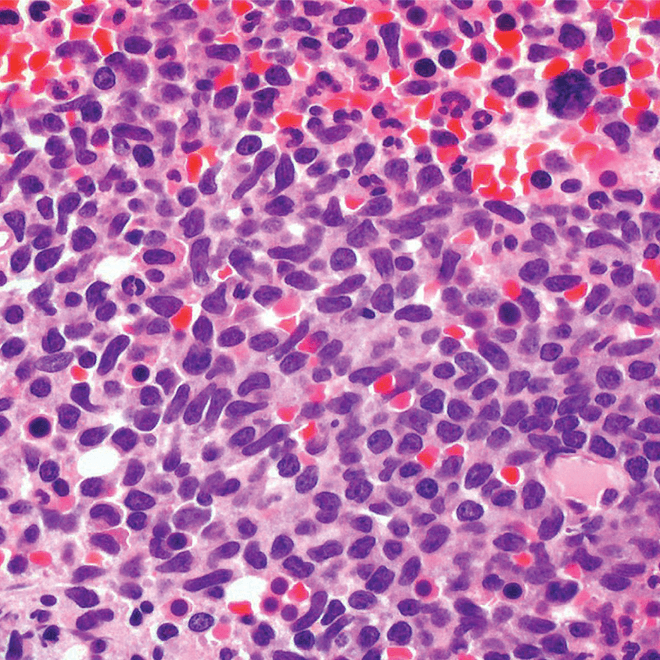
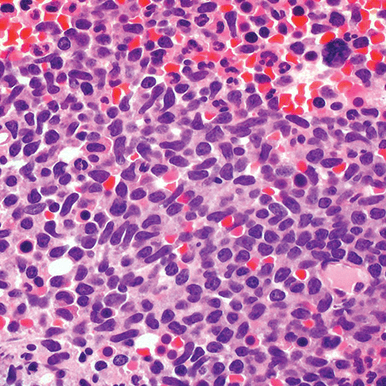
- Bone marrow
- Core biopsy showing diffuse infiltrate by BPDCN (H&E stain, x600).11
-
Reprinted by permission of SAGE Publications, Inc.
Main morphologic features of BPDCN biopsy
- Diffuse, monomorphic infiltrate1
- Medium-sized blast cells with irregular nuclei1
- Fine chromatin1
- At least 1 small nucleolus1
- Malignant BPDCN cells do not typically infiltrate the epidermis4
BPDCN with low-density infiltrate may mimic an inflammatory condition4
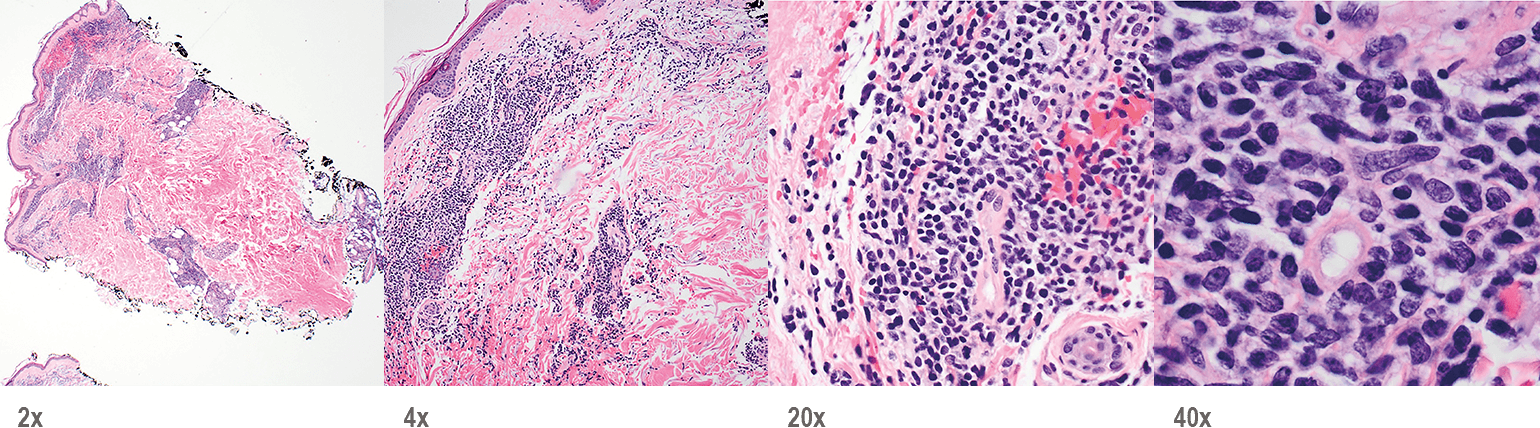
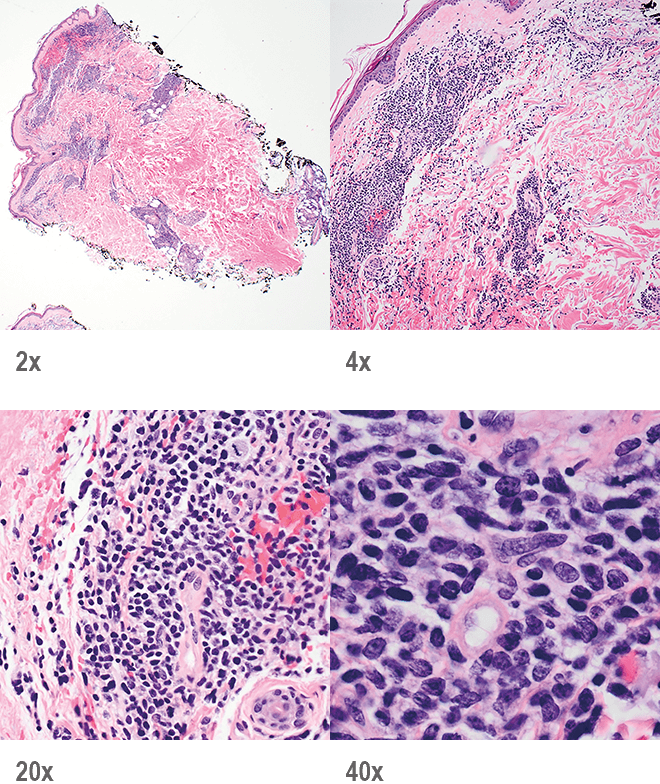
Image provided by Sheeja Pullarkat, MD.
- Cutaneous cases with minimal involvement show periadnexal and perivascular infiltrate, clustering in the superficial to mid dermis12,13
- Cytology, in association with flow cytometry immunophenotyping and clinical history, can help obtain an accurate diagnosis of BPDCN14
To help with a timely and accurate diagnosis, consider including CD123, CD4, and CD56 in early diagnostic panels1,2
BPDCN, blastic plasmacytoid dendritic cell neoplasm; H&E, hematoxylin and eosin; TdT, terminal deoxynucleotidyl transferase.
- References:
- Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188-202.
- Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239-246.
- Laribi K, et al. Blastic plasmacytoid dendritic cell neoplasm: from origin of the cell to targeted therapies. Biol Blood Marrow Transplant. 2016;22(8):1357-1367.
- Facchetti F, et al. Neoplasms derived from plasmacytoid dendritic cells. Mod Pathol. 2016;29(2):98-111.
- Frankel AE, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385-392.
- Ceribelli M, et al. A druggable TCF4- and BRD4-dependent transcriptional network sustains malignancy in blastic plasmacytoid dendritic cell neoplasm. Cancer Cell. 2016;30(5):764-778.
- Cronin DMP, et al. Immunophenotypic analysis of myeloperoxidase-negative leukemia cutis and blastic plasmacytoid dendritic cell neoplasm. Am J Clin Pathol. 2012;137(3):367-376.
- Sangle NA, et al. Optimized immunohistochemical panel to differentiate myeloid sarcoma from blastic plasmacytoid dendritic cell neoplasm. Mod Pathol. 2014;27(8):1137-1143.
- Deotare U, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: 10-color flow cytometry diagnosis and HyperCVAD therapy. Am J Hematol. 2016;91(3):283-286.
- Garnache-Ottou F, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145(5):624-636.
- Riaz W, et al. Blastic plasmacytoid dendritic cell neoplasm: update on molecular biology, diagnosis, and therapy. Cancer Control. 2014;21(4):279-289.
- Reichard KK. Blastic plasmacytoid dendritic cell neoplasm: how do you distinguish it from acute myeloid leukemia? Surg Pathol Clin. 2013;6(4):743-765.
- Sullivan JM, Rizzieri DA. Treatment of blastic plasmacytoid dendritic cell neoplasm. Hematology Am Soc Hematol Educ Program. 2016;2016(1):16-23.
- Ferreira J, et al. Cytomorphological features of blastic plasmacytoid dendritic cell neoplasm on FNA and cerebrospinal fluid cytology: a review of 6 cases. Cancer Cytopathol. 2016;124(3):196-202.




