The first cycle of ELZONRIS should be administered in an inpatient setting, while the subsequent cycles can be administered in the inpatient setting or a suitable outpatient ambulatory care setting equipped with appropriate observation for patients with hematopoietic malignancies undergoing treatment.1

Prepare to observe patients through at least 24 hours after the last infusion during cycle 1 in the inpatient setting1

In cycle two and beyond, prepare to observe patients for at least 4 hours following each infusion for any adverse events1
Factors to consider before dose administration1


Prior to administration, ELZONRIS is diluted to 100 micrograms per milliliter according to the patient’s weight


Do not reuse excess ELZONRIS. Any excess material should be thrown away immediately following infusion


Administer ELZONRIS until disease progression or unacceptable toxicity occurs. Do not administer as an IV push or bolus


Refer to the Package Insert for a complete list of Warnings and Precautions and recommended dose modifications
Pre-medicate patients ~60 minutes prior to each infusion with1:
- H1-histamine antagonist (eg, diphenhydramine hydrochloride)
- Acetaminophen (paracetamol)
- Corticosteroid (eg, 50 mg IV methylprednisolone or equivalent)
- H2-histamine antagonist (eg, famotidine)
IV, intravenous
- Reference:
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.
This page is intended to be a practical guide. Use the checklists to keep track of the steps. Please note that selections are not stored. Your selections will be lost if you refresh/close the page.
Steps for dose administration1:
|
Step 1 Prior to infusion, check the patient’s ID band, establish venous access, and maintain with sterile 0.9% Sodium Chloride Injection, USP. 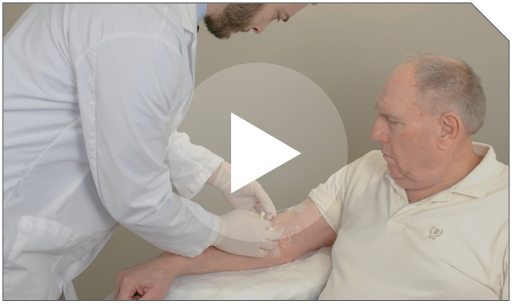
|
|
Step 2 Insert the syringe containing the diluted ELZONRIS dose into the programmable syringe pump, following the pump’s instructions. Please refer to the manufacturer’s instructions for your pump to ensure proper use. 
|
|
Step 3 The total infusion time will be controlled using a programmable pump to deliver the entire diluted ELZONRIS dose over 15 minutes. This time frame includes the 0.9% Sodium Chloride Injection, USP flush. 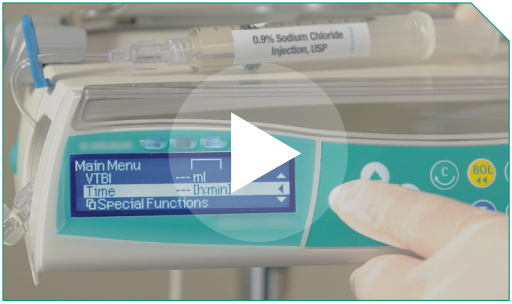
|
|
Step 4 The pump must have settings that enable you to input the total dose amount required for infusion and the time required for delivery. 
|
|
Step 5 Before you connect the administration setup to the patient’s IV line, determine where you will place the 0.9% Sodium Chloride Injection, USP flush so it can be conveniently accessed upon completion of the diluted ELZONRIS dose. 
|
|
Step 6 Attach the outlet of the in-line filter to the Y-connector of the patient’s IV line. 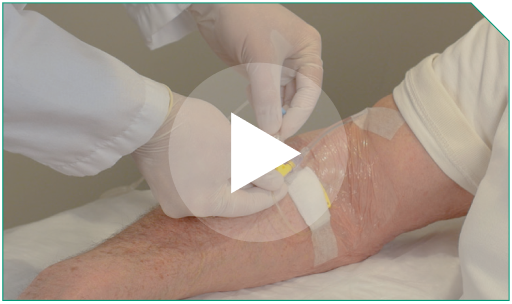
|
|
Step 7 Stop the patient’s running 0.9% Sodium Chloride Injection, USP line by clamping it and then start the infusion pump. 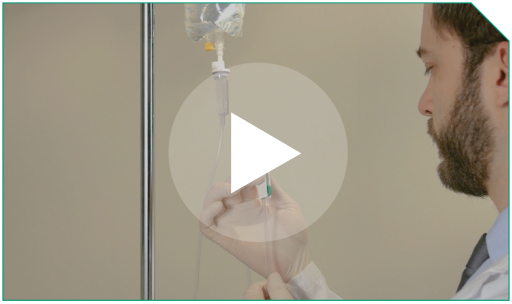
Run the infusion syringe pump until the diluted ELZONRIS-filled syringe is empty. This will take less than 15 minutes. 
|
|
Step 8 A healthcare professional should closely observe the patient during infusion for any potential adverse or site reactions. 
|
|
Step 9 Depending on the type of pump you are using, alarms may sound to indicate that the syringe is almost empty. Visually verify that the syringe is completely empty. 
|
|
Step 10 When the diluted ELZONRIS-filled syringe is completely empty, remove it from the pump, following the pump’s instructions. Do not turn off the pump. 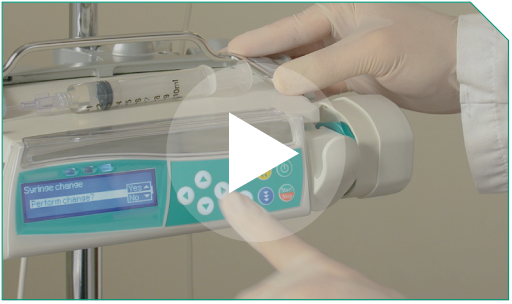
Clamp the ELZONRIS side of the Y-connector. 
|
|
Step 11 Clamp the ELZONRIS side of the Y-connector. 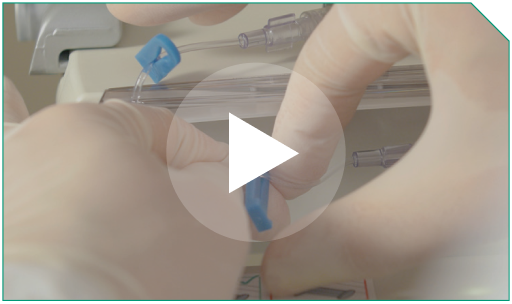
|
|
Step 12 Open the clamp on the 0.9% Sodium Chloride Injection, USP flush side of the Y-connector. 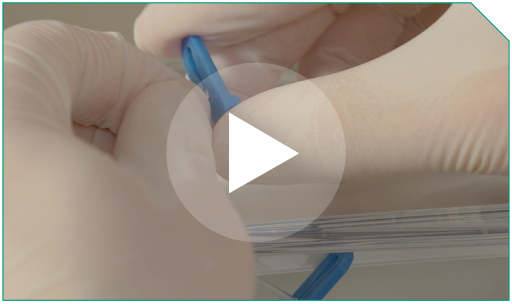

|
|
Step 13 Place the 0.9% Sodium Chloride Injection, USP flush syringe in the programmable pump. 
Then resume infusion via the programmable pump at the pre-specified flow rate to completely deliver the diluted ELZONRIS dose remaining in the microbore tubing line. 
|
|
Step 14 You should ensure that the full recommended dose of diluted ELZONRIS has been sufficiently administered during the 15-minute time frame. 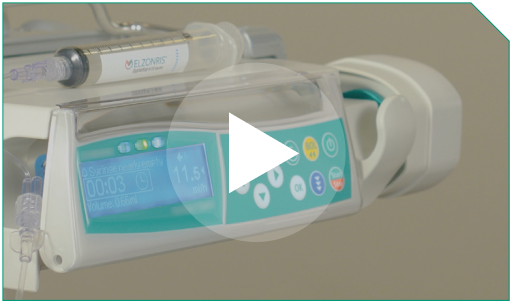
|
|
Step 15 When that has happened, remove the 0.9% Sodium Chloride Injection, USP flush syringe from the programmable pump. The 0.9% Sodium Chloride Injection, USP flush syringe will not be emptied entirely at the end of the 15-minute infusion, since it is just intended to push the remaining ELZONRIS dose out of the infusion line to complete dose delivery. 
Power off the pump by following the pump’s instructions. 
|
|
Step 16 Disconnect the in-line filter port from the patient’s IV line. Prepare to monitor the patient through at least 24 hours after the last infusion during Cycle 1 in the inpatient setting, and at least 4 hours following each infusion in the subsequent cycles for any adverse events. 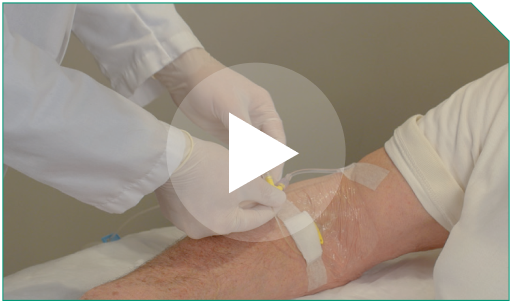
|
Step 1 |
Prior to infusion, check the patient’s ID band, establish venous access, and maintain with sterile 0.9% Sodium Chloride Injection, USP. |  |
|
Step 2 |
Insert the syringe containing the diluted ELZONRIS dose into the programmable syringe pump, following the pump’s instructions. Please refer to the manufacturer’s instructions for your pump to ensure proper use. |  |
|
Step 3 |
The total infusion time will be controlled using a programmable pump to deliver the entire diluted ELZONRIS dose over 15 minutes. This time frame includes the 0.9% Sodium Chloride Injection, USP flush. |  |
|
Step 4 |
The pump must have settings that enable you to input the total dose amount required for infusion and the time required for delivery. |  |
|
Step 5 |
Before you connect the administration setup to the patient’s IV line, determine where you will place the 0.9% Sodium Chloride Injection, USP flush so it can be conveniently accessed upon completion of the diluted ELZONRIS dose. |  |
|
Step 6 |
Attach the outlet of the in-line filter to the Y-connector of the patient’s IV line. |  |
|
Step 7 |
Stop the patient’s running 0.9% Sodium Chloride Injection, USP line by clamping it and then start the infusion pump.  Run the infusion syringe pump until the diluted ELZONRIS-filled syringe is empty. This will take less than  |
||
Step 8 |
A healthcare professional should closely observe the patient during infusion for any potential adverse or site reactions. |  |
|
Step 9 |
Depending on the type of pump you are using, alarms may sound to indicate that the syringe is almost empty. Visually verify that the syringe is completely empty. |  |
|
Step 10 |
When the diluted ELZONRIS-filled syringe is completely empty, remove it from the pump, following the pump’s instructions. Do not turn off the pump.   |
||
Step 11 |
Clamp the ELZONRIS side of the Y-connector. |  |
|
Step 12 |
Open the clamp on the 0.9% Sodium Chloride Injection, USP flush side of the Y-connector.   |
||
Step 13 |
Place the 0.9% Sodium Chloride Injection, USP flush syringe in the programmable pump.  Then resume infusion via the programmable pump at the pre-specified flow rate to completely deliver the diluted ELZONRIS dose remaining in the microbore tubing line.  |
||
Step 14 |
You should ensure that the full recommended dose of diluted ELZONRIS has been sufficiently administered during the 15-minute time frame. |  |
|
Step 15 |
When that has happened, remove the 0.9% Sodium Chloride Injection, USP flush syringe from the programmable pump. The 0.9% Sodium Chloride Injection, USP flush syringe will not be emptied entirely at the end of the 15-minute infusion, since it is just intended to push the remaining ELZONRIS dose out of the infusion line to complete dose delivery.  Power off the pump by following the pump’s instructions.  |
||
Step 16 |
Disconnect the in-line filter port from the patient’s IV line. Prepare to monitor the patient through at least 24 hours after the last infusion during Cycle 1 in the inpatient setting, and at least 4 hours following each infusion in the subsequent cycles for any adverse events. |
 |
|
IV, intravenous.
- Reference:
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.
Well-defined dose modification guidelines have been established for ELZONRIS administration1
| Parameter | Severity criteria | Dosage modification |
|---|---|---|
| Serum albumin | Serum albumin |
See CLS Management Guidelines |
| Body weight | Body weight increase |
See CLS Management Guidelines |
| AST or ALT | ALT or AST increase >5 times the upper limit of normal | Withhold ELZONRIS until transaminase elevations are |
| Serum creatinine | Serum creatinine |
Withhold ELZONRIS until serum creatinine resolves to |
| Systolic blood pressure | Systolic blood pressure |
Withhold ELZONRIS until systolic blood pressure is |
| Heart rate | Heart rate |
Withhold ELZONRIS until heart rate is |
| Body temperature | Body temperature ≥38°C | Withhold ELZONRIS until body temperature is <38°C |
| Hypersensitivity reactions | Mild or moderate | Withhold ELZONRIS until resolution of any mild or moderate hypersensitivity reaction. Resume ELZONRIS at the same infusion rate |
| Severe or life-threatening | Discontinue ELZONRIS permanently |
Ensure the patient meets all requirements prior to ELZONRIS administration1
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLS, capillary leak syndrome.
- Reference:
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.








 Previous
Previous