- To get started, lay out all your components to ensure that you have everything you need. Your components may vary depending on their manufacturer. However, all the components pictured here are required for the assembly of the ELZONRIS administration system. All components must be sterile.
Components for Dose Preparation

Before initiating the ELZONRIS dose preparation process, make sure you have everything you need, including your institution’s preferred syringe pump.
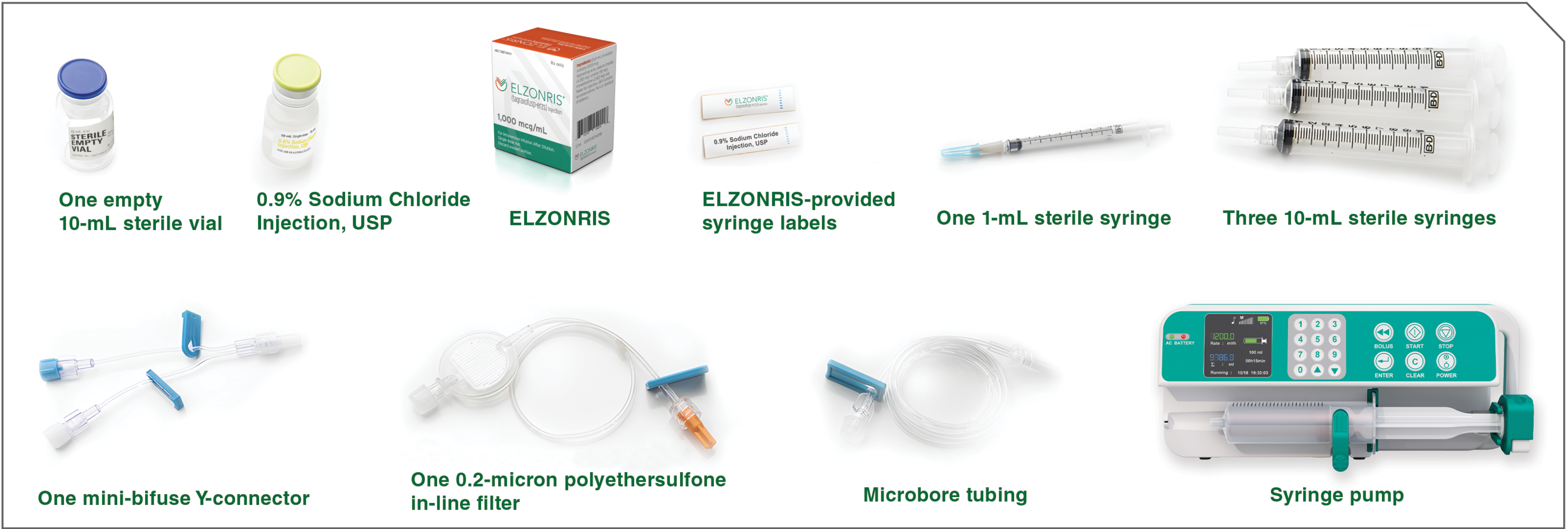
Dose preparation components
- Please ensure that all of the following components are available prior to thawing ELZONRIS1:
|
Empty 10-mL sterile vial 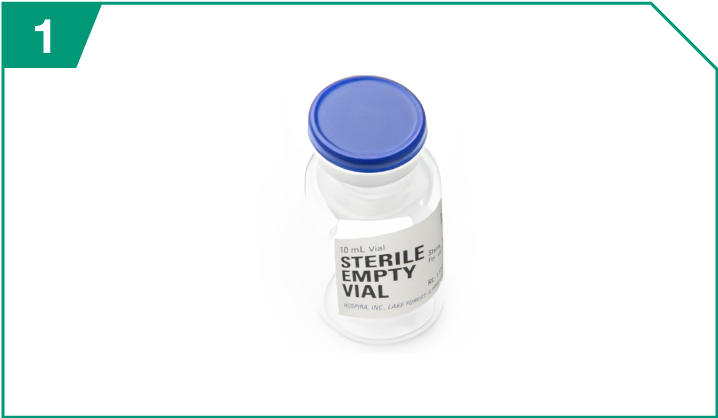
|
|
Mini-bifuse Y-connector 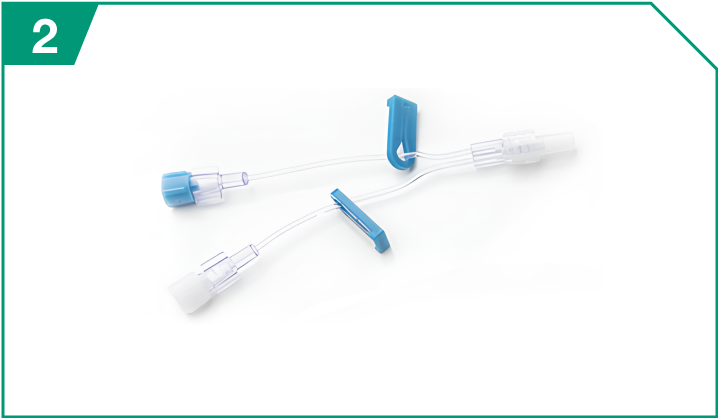
|
|
0.2-micron polyethersulfone in-line filter 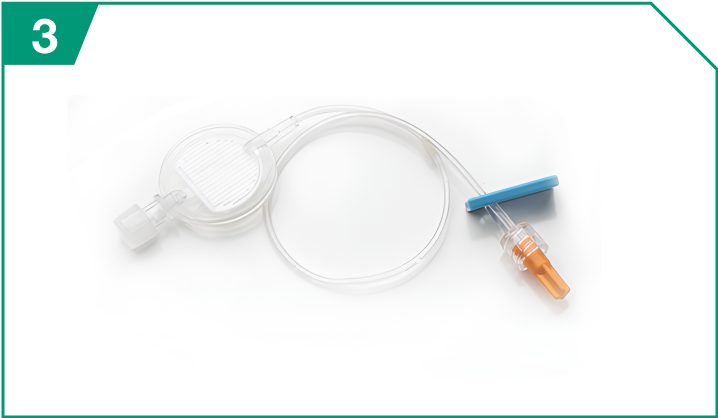
|
|
Microbore tubing 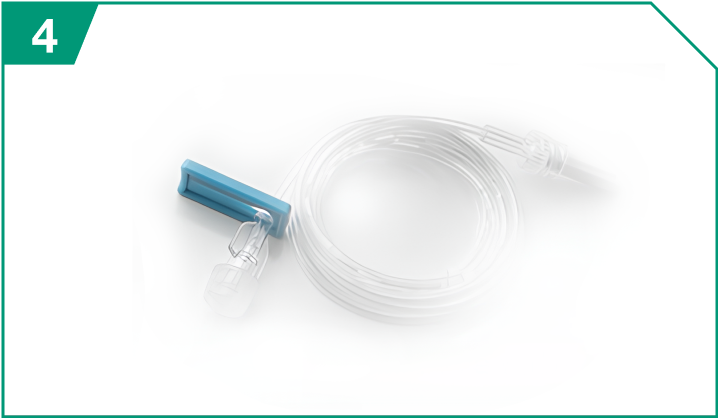
|
|
ELZONRIS-provided syringe label 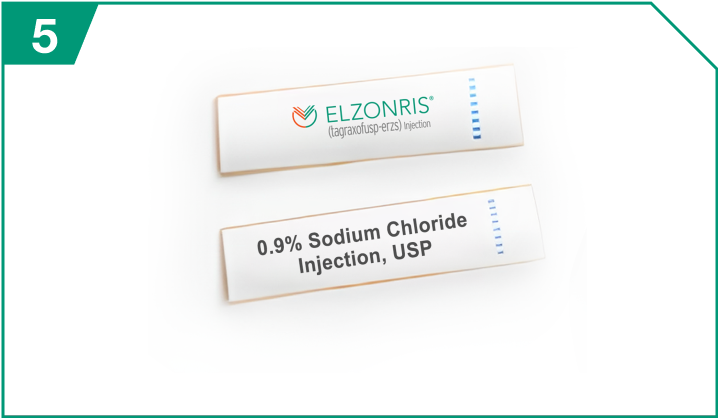
|
|
Three 10-mL sterile syringes 
|
|
One 1-mL sterile syringe 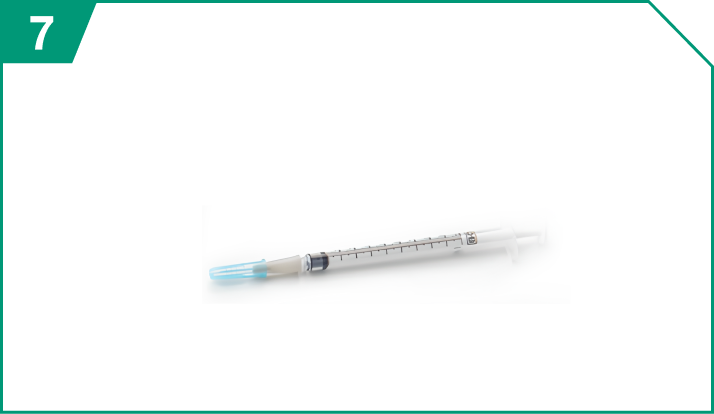
|
|
0.9% Sodium Chloride Injection, USP 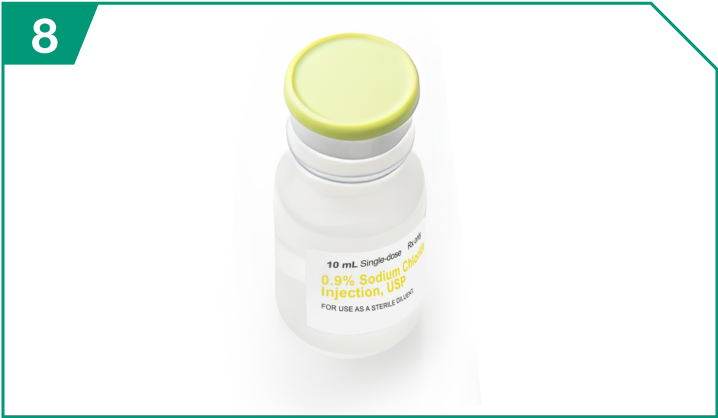
|
|
ELZONRIS 
|
|
Infusion syringe pump 
|
| Empty 10-mL sterile vial |  |
|
| Mini-bifuse Y-connector |  |
|
| 0.2-micron polyethersulfone in-line filter |  |
|
| Microbore tubing |  |
|
| ELZONRIS-provided syringe label |  |
|
| Three 10-mL sterile syringes |  |
|
| One 1-mL sterile syringe |  |
|
| 0.9% Sodium Chloride Injection, USP |  |
|
| ELZONRIS |  |
|
| Infusion syringe pump |  |
Calculate the required dose based on the recommended dosage of ELZONRIS1*:
Patient’s weight
Calculated Dose: 12 mcg/kg X patient’s body weight (kg) =
*Ensure that the required components are available to support the dilution of a second vial, if the calculated dose based on the patient's weight exceeds 10 mL of ELZONRIS.1
ELZONRIS Injection Instructions for Intravenous Use1

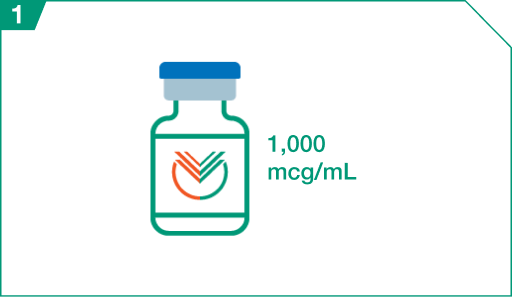
ELZONRIS injection for intravenous use is a preservative-free, sterile, clear, colorless,
1,000 mcg/mL solution supplied in a single-dose glass vial.
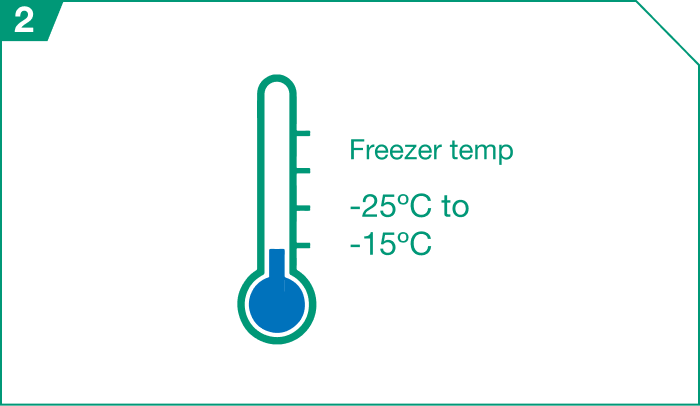
ELZONRIS vials should be stored in a freezer between -25º Celsius and -15º Celsius (or between -13º Fahrenheit and 5º Fahrenheit) and protected from light.
Prior to thawing, the patient’s vital signs, serum albumin, transaminases, and serum creatinine levels should be checked to ensure patient is able to receive ELZONRIS.
To prepare the first dose, first calculate the required volume of diluted ELZONRIS based on the patient’s body weight and then determine what components you need for preparation.
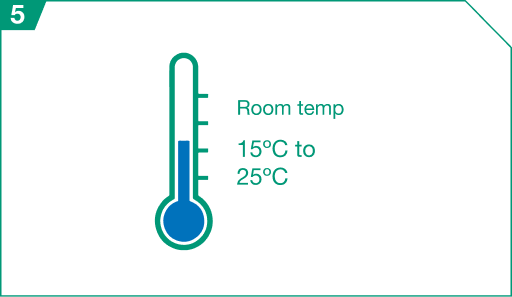
Prior to dose preparation, thaw at room temperature between 15º Celsius and 25º Celsius (or 59º Fahrenheit and 77º Fahrenheit) for 15 to 30 minutes in the original carton, and verify thaw visually.
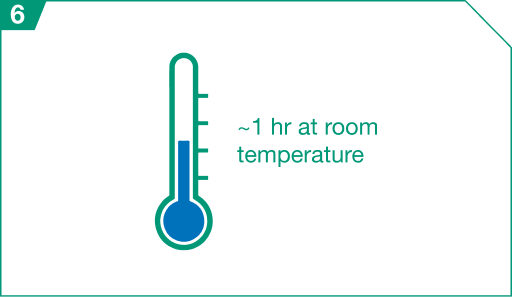
Thawed vials may be held at room temperature for approximately 1 hour prior to dosage preparation.

Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.

Thawed ELZONRIS appearance should be a clear, colorless liquid that may contain a few white to translucent particles. Do not force thaw. Do not refreeze.

Administer ELZONRIS within 4 hours of completing the dose preparation. During this
4-hour window, the prepared dose should remain at room temperature.

Use aseptic technique for preparation of ELZONRIS dose.
- Reference:
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.
Prepare ELZONRIS under a laminar flow hood using aseptic technique.
This is one example of an assembled administration system:

Some of the components may look slightly different depending on the manufacturer. If the pharmacy did not include one or more of these components, such as a full syringe for the flush, you should procure your own.
This page is intended to be a practical guide. Use the checklists to keep track of the steps. Please note that selections are not stored. Your selections will be lost if you refresh/close the page.
Steps for dose preparation1:
|
Step 1 Using a sterile 10-mL syringe, withdraw 9 mL 0.9% Sodium Chloride Injection USP, and transfer into the empty 10-mL sterile vial. 
|
|
Step 2 Next, gently swirl the ELZONRIS vial to mix the contents. 
|
|
Step 3 Remove the cap and, using a sterile 1-mL syringe, withdraw 1 mL of thawed ELZONRIS from the product vial. 
|
|
Step 4 Transfer the 1 mL of ELZONRIS into the 10-mL vial containing the 9 mL of 0.9% Sodium Chloride Injection, USP. 
|
|
Step 5 To ensure adequate mixing of the diluted ELZONRIS, gently invert the vial containing the 0.9% Sodium Chloride Injection, USP and ELZONRIS at least 3 times. Do not shake vigorously; avoid foaming. 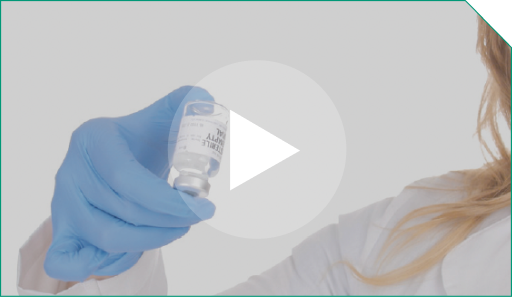
|
|
Step 6 Following dilution, the final concentration of ELZONRIS is 100 mcg/mL. 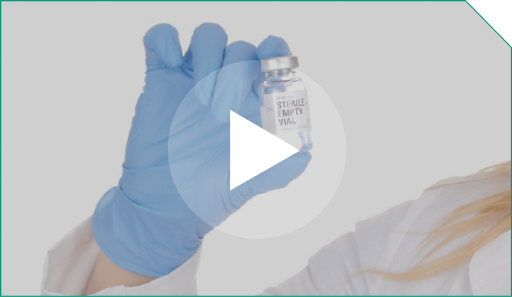
|
|
Step 7 The recommended dose of ELZONRIS is 12 mcg/kg diluted to 100 mcg/mL. If more than 10 mL of ELZONRIS is calculated based on the patient’s weight, ensure that the required components are available to support the dilution of a second vial and use a 20-mL sterile syringe when delivering ELZONRIS. 
|
|
Step 8 Using a new 10-mL syringe, withdraw the correct dosage volume from the vial containing the diluted ELZONRIS. 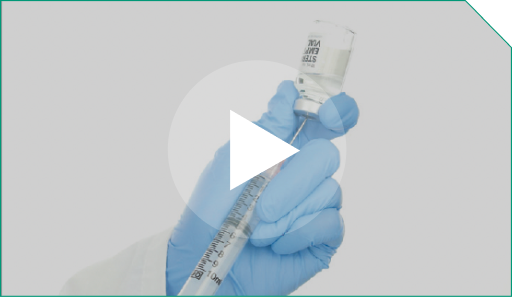
|
|
Step 9 Cap and label the ELZONRIS-filled syringe with the ELZONRIS-provided label, and set it aside. Each label will have a unique item code number. 
|
|
Step 10 Next, take a new 10-mL sterile syringe... 
and fill it with at least 3 mL of 0.9% Sodium Chloride Injection, USP. This will be the 0.9% Sodium Chloride Injection, USP flush syringe. The 0.9% Sodium Chloride Injection, USP flush will be used to push out the full required dose of ELZONRIS to the patient at the end of the infusion. 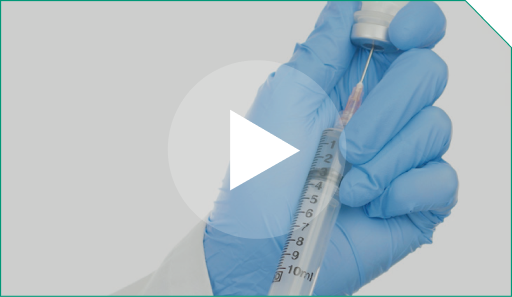
|
|
Step 11 Cap and label this syringe with the 0.9% Sodium Chloride Injection, USP provided label. 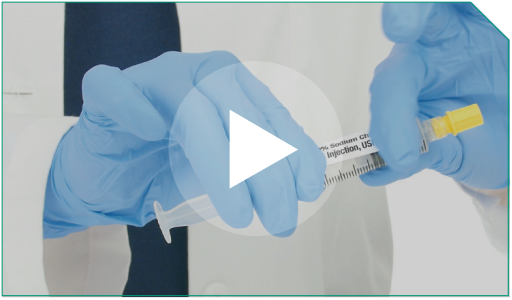
|
|
Step 12 Attach the ELZONRIS-filled syringe to one arm of the Y-connector. 
Attach the 0.9% Sodium Chloride Injection, USP syringe to the other arm of the Y-connector. Clamp off both arms until needed. 
|
|
Step 13 Connect the terminal end of the Y-connector to the microbore tubing. 
|
|
Step 14 Remove the cap from the supply side of the 0.2-micron filter and… 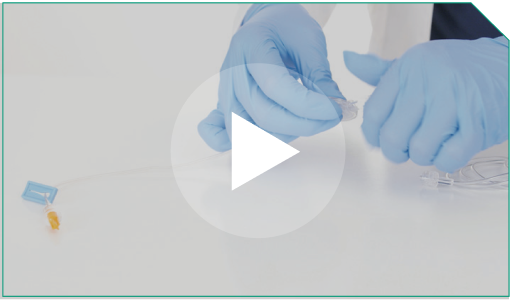
attach it to the terminal end of the microbore tubing. 
|
|
Step 15 All components are now attached. 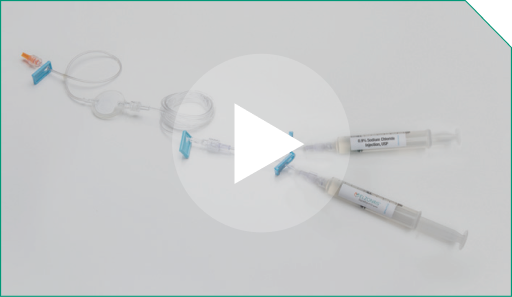
|
|
Step 16 Next, prime the 0.9% Sodium Chloride Injection, USP and ELZONRIS-filled lines. Unclamp the arm of the Y-connector connected to the 0.9% Sodium Chloride Injection, USP flush syringe. 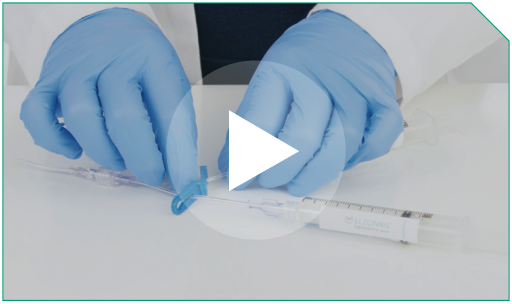
Prime the Y-connector up to the intersection. Do not prime the full infusion set with 0.9% Sodium Chloride Injection, USP. Re-clamp the Y-connector line of the 0.9% Sodium Chloride Injection flush arm. 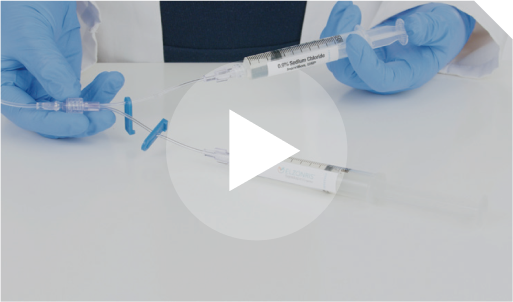
|
|
Step 17 Remove the cap from the opposite side of the in-line filter and set it aside. 
Next, prime the ELZONRIS side of the Y-connector, the microbore tubing, and the in-line filter. 
|
|
Step 18 Put the cap back onto the in-line filter to ensure that ELZONRIS will not leak out. 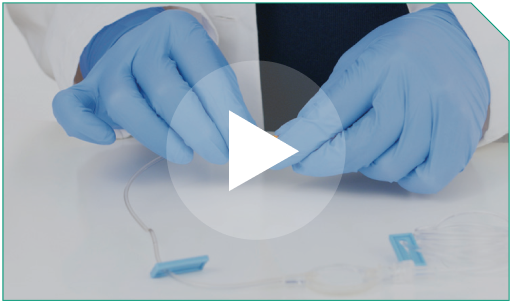
|
|
Step 19 The infusion set is now ready for delivery for dose administration. Finally, label the administration setup according to your institutional guidelines. 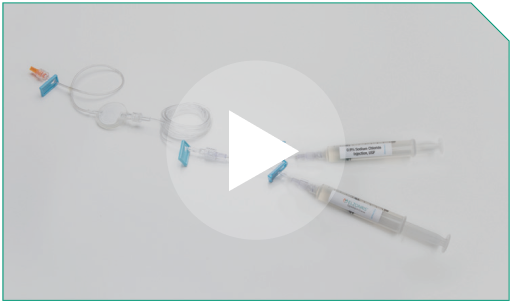
|
Step 1 |
Using a sterile 10-mL syringe, withdraw 9 mL 0.9% Sodium Chloride Injection USP, and transfer into the empty 10-mL sterile vial. |  |
|
Step 2 |
Next, gently swirl the ELZONRIS vial to mix the contents. |  |
|
Step 3 |
Remove the cap and, using a sterile
1-mL syringe, withdraw 1 mL of thawed ELZONRIS from the product vial. |
 |
|
Step 4 |
Transfer the 1 mL of ELZONRIS into the 10-mL vial containing the 9 mL of 0.9% Sodium Chloride Injection, USP. |  |
|
Step 5 |
To ensure adequate mixing of the diluted ELZONRIS, gently invert the vial containing the 0.9% Sodium Chloride Injection, USP and ELZONRIS at least 3 times. Do not shake vigorously; avoid foaming. |  |
|
Step 6 |
Following dilution, the final concentration of ELZONRIS is 100 mcg/mL. |
 |
|
Step 7 |
The recommended dose of ELZONRIS is 12 mcg/kg diluted to 100 mcg/mL. If more than 10 mL of ELZONRIS is calculated based on the patient’s weight, ensure that the required components are available to support the dilution of a second vial and use a 20-mL sterile syringe when delivering ELZONRIS. |  |
|
Step 8 |
Using a new 10-mL syringe, withdraw the correct dosage volume from the vial containing the diluted ELZONRIS. |  |
|
Step 9 |
Cap and label the ELZONRIS-filled syringe with the ELZONRIS-provided label, and set it aside. Each label will have a unique item code number. |  |
|
Step 10 |
Next, take a new 10-mL sterile syringe...  and fill it with at least 3 mL of 0.9% Sodium Chloride Injection, USP. This will be the 0.9% Sodium Chloride Injection, USP flush syringe. The 0.9% Sodium Chloride Injection, USP flush will be used to push out the full required dose of ELZONRIS to the patient at the end of the infusion.  |
||
Step 11 |
Cap and label this syringe with the 0.9% Sodium Chloride Injection, USP provided label. |  |
|
Step 12 |
Attach the ELZONRIS-filled syringe to one arm of the Y-connector.  Attach the 0.9% Sodium Chloride Injection, USP syringe to the other arm of the Y-connector. Clamp off both arms until needed.  |
||
Step 13 |
Connect the terminal end of the Y-connector to the microbore tubing. |  |
|
Step 14 |
Remove the cap from the supply side of the 0.2-micron filter and…  attach it to the terminal end of the microbore tubing.  |
||
Step 15 |
All components are now attached. |  |
|
Step 16 |
Next, prime the 0.9% Sodium Chloride Injection, USP and ELZONRIS-filled lines. Unclamp the arm of the Y-connector connected to the 0.9% Sodium Chloride Injection, USP flush syringe.  Prime the Y-connector up to the intersection. Do not prime the full infusion set with 0.9% Sodium Chloride Injection, USP. Re-clamp the Y-connector line of the 0.9% Sodium Chloride Injection flush arm.  |
||
Step 17 |
Remove the cap from the opposite side of the in-line filter and set it aside.  Next, prime the ELZONRIS side of the Y-connector, the microbore tubing, and the in-line filter.  |
||
Step 18 |
Put the cap back onto the in-line filter to ensure that ELZONRIS will not leak out. |  |
|
Step 19 |
The infusion set is now ready for delivery for dose administration. Finally, label the administration setup according to your institutional guidelines. |  |
|
Ensure the patient meets all requirements prior to ELZONRIS preparation1
- Reference:
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.








 Previous
Previous