Patient Profiles
Healthcare Professionals

Meet the patients

Ellie
a candidate
for HSCT

Ian
may not be eligible
for HSCT

Ralph
whose BPDCN was
previously treated
Ellie may benefit from ELZONRIS to bridge to HSCT

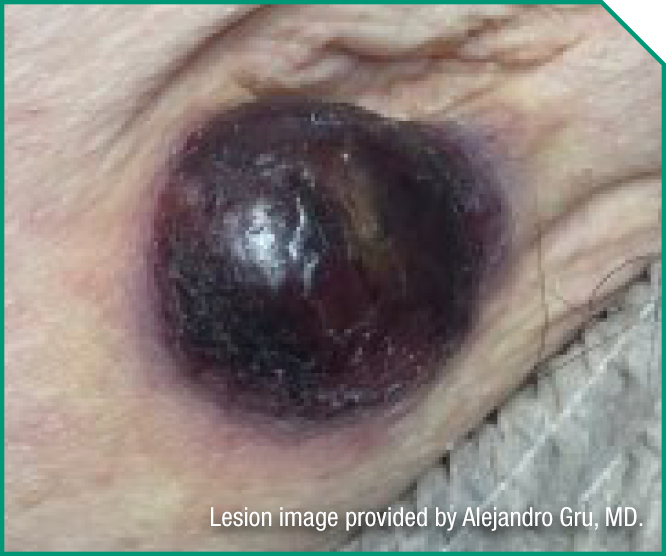
Ellie is a 45-year-old avid cyclist and proud mother to 2 teenagers. She has had no prior treatment for her BPDCN.
Medical history
- No peripheral blood, bone marrow, lymph node, or viscera involvement
- ECOG status: 0
- Serum albumin: 5.0 g/dL
“I first noticed a dark purple lump on my stomach and waited 2 weeks to see if it would go away. When it didn't, I saw my dermatologist, who ran a bunch of tests and eventually told me that I had something called BPDCN.
She then referred me to a hematologist-oncologist. ”
What would you do to bridge a patient like Ellie to HSCT?
ELZONRIS may help extend Ellie's survival1
lan may not be eligible for transplant, but still may need help achieving remission

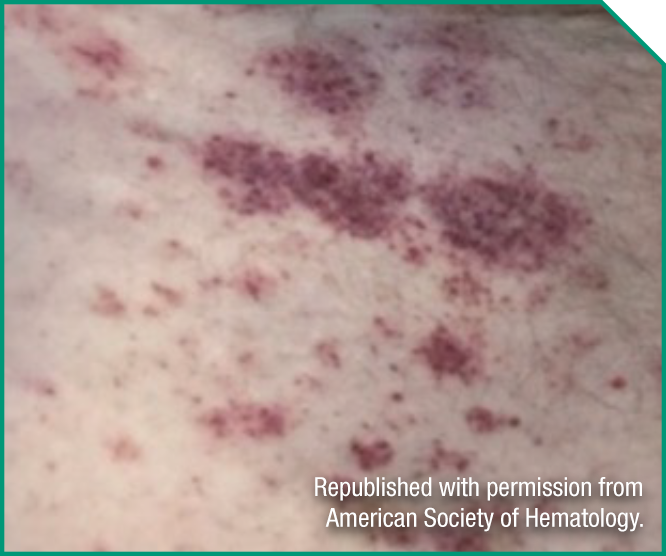
lan is a 68-year-old doting husband and grandfather. He has had no prior treatment for his BPDCN.
Medical history
- Peripheral blood and lymph node involvement
- ECOG status: 2
- Serum albumin: 3.5 g/dL
“My wife spotted a patch of bruises on my shoulder, so we went to
see my PCP. She was
concerned about the results of my blood
work
and sent me to a hematologist-oncologist, who did a biopsy.
After weeks of anxiously waiting,
I was diagnosed with BPDCN. ”
What would you do to achieve remission in a patient like lan?
With ELZONRIS, remission may be possible in your patients like lan1,2
ELZONRIS may help previously treated Ralph achieve rapid and durable remission1,2

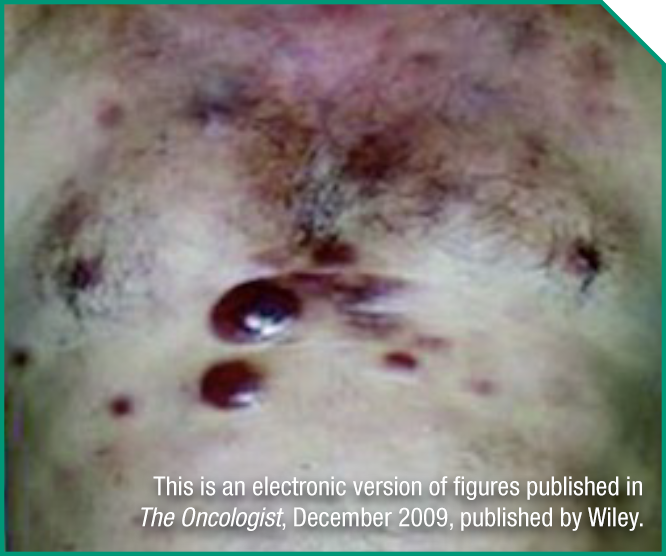
Ralph is an 80-year-old retired salesman and active member of a bowling league. He was previously treated with HCVAD and relapsed 6 months after treatment.
Medical history
- Bone marrow and viscera involvement
- ECOG status: 1
- Serum albumin: 4.0 g/dL
“I went to my dermatologist after spotting purple lumps on my chest. He referred me to a hematologist-oncologist who ran some tests, including a biopsy, and diagnosed me with BPDCN. I was hopeful that chemo would work for me, but I'm devastated to have relapsed. I don't know what my future holds. ”
What would you do to achieve remission in a patient like Ralph?
There's hope for patients like Ralph to achieve remission with ELZONRIS1,2
BPDCN, blastic plasmacytold dendritic cell neoplasm; ECOG, Eastern Cooperative Oncology Group; HCVAD, hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone; HSCT, hematopoietic stem cell transplantation; PCP, primary care physician.
in the treatment of BPDCN1,3
- References:
- Pemmaraju N, et al. Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2022;40(26):3032-3036.
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.
- Study of tagraxofusp reports 90 percent response rate for deadly blood cancer with no prior available therapies. MD Anderson Newsroom. Published April 24, 2019. Accessed December 12, 2023. https://www.mdanderson.org/newsroom/study-of-tagraxofusp-reports-90-percent-response-rate-for-deadly.h00-159302256.html
INDICATION
- ELZONRIS is a CD123-directed cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and in pediatric patients 2 years and older
IMPORTANT SAFETY INFORMATION
Boxed WARNING: CAPILLARY LEAK SYNDROME
- Capillary Leak Syndrome (CLS), which may be life-threatening or fatal, can occur in patients receiving ELZONRIS. Monitor for signs and symptoms of CLS and take actions as recommended.
WARNINGS AND PRECAUTIONS
Capillary Leak Syndrome
- Capillary leak syndrome (CLS), including life-threatening and fatal cases, has been reported among patients treated with ELZONRIS. In patients receiving ELZONRIS in clinical trials, the overall incidence of CLS was 53% (65/122), including Grade 1 or 2 in 43% (52/122) of patients, Grade 3 in 7% (8/122) of patients, Grade 4 in 1% (1/122) of patients, and four fatalities (3%). The median time to onset was 4 days (range-1 to 46 days), and all but 5 patients experienced an event in Cycle 1.
- Before initiating therapy with ELZONRIS, ensure that the patient has adequate cardiac function and serum albumin is greater than or equal to 3.2 g/dL. During treatment with ELZONRIS, monitor serum albumin levels prior to the initiation of each dose of ELZONRIS and as indicated clinically thereafter, and assess patients for other signs or symptoms of CLS, including weight gain, new onset or worsening edema, including pulmonary edema, hypotension or hemodynamic instability.
Hypersensitivity Reactions
- ELZONRIS can cause severe hypersensitivity reactions. In patients receiving ELZONRIS in clinical trials, hypersensitivity reactions were reported in 43% (53/122) of patients treated with ELZONRIS and were Grade ≥ 3 in 7% (9/122). Manifestations of hypersensitivity reported in
≥ 5% of patients include rash, pruritus, and stomatitis. Monitor patients for hypersensitivity reactions during treatment with ELZONRIS. Interrupt ELZONRIS infusion and provide supportive care as needed if a hypersensitivity reaction should occur.
Hepatotoxicity
- Treatment with ELZONRIS was associated with elevations in liver enzymes. In patients receiving ELZONRIS in clinical trials, elevations in ALT occurred in 79% (96/122) and elevations in AST occurred in 76% (93/122). Grade 3 ALT elevations were reported in 26% (32/122) of patients. Grade 3 AST elevations were reported in 30% (36/122) and Grade 4 AST elevations were reported in 3% (4/122) of patients. Elevated liver enzymes occurred in the majority of patients in Cycle 1 and were reversible following dose interruption.
- Monitor alanine aminotransferase (ALT) and aspartate aminotransferase (AST) prior to each infusion with ELZONRIS. Withhold ELZONRIS temporarily if the transaminases rise to greater than 5 times the upper limit of normal and resume treatment upon normalization or when resolved.
ADVERSE REACTIONS:
Most common adverse reactions (incidence
Please see Full Prescribing Information, including Boxed WARNING.
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
IMPORTANT SAFETY INFORMATION
INDICATION
- ELZONRIS is a CD123-directed cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and in pediatric patients 2 years and older
IMPORTANT SAFETY INFORMATION
Boxed WARNING: CAPILLARY LEAK SYNDROME
- Capillary Leak Syndrome (CLS), which may be life-threatening or fatal, can occur in patients receiving ELZONRIS. Monitor for signs and symptoms of CLS and take actions as recommended.
WARNINGS AND PRECAUTIONS
Capillary Leak Syndrome
- Capillary leak syndrome (CLS), including life-threatening and fatal cases, has been reported among patients treated with ELZONRIS. In patients receiving ELZONRIS in clinical trials, the overall incidence of CLS was 53% (65/122), including Grade 1 or 2 in 43% (52/122) of patients, Grade 3 in 7% (8/122) of patients, Grade 4 in 1% (1/122) of patients, and four fatalities (3%). The median time to onset was 4 days (range-1 to 46 days), and all but 5 patients experienced an event in Cycle 1.
- Before initiating therapy with ELZONRIS, ensure that the patient has adequate cardiac function and serum albumin is greater than or equal to 3.2 g/dL. During treatment with ELZONRIS, monitor serum albumin levels prior to the initiation of each dose of ELZONRIS and as indicated clinically thereafter, and assess patients for other signs or symptoms of CLS, including weight gain, new onset or worsening edema, including pulmonary edema, hypotension or hemodynamic instability.
Hypersensitivity Reactions
- ELZONRIS can cause severe hypersensitivity reactions. In patients receiving ELZONRIS in clinical trials, hypersensitivity reactions were reported in 43% (53/122) of patients treated with ELZONRIS and were Grade ≥ 3 in 7% (9/122). Manifestations of hypersensitivity reported in
≥ 5% of patients include rash, pruritus, and stomatitis. Monitor patients for hypersensitivity reactions during treatment with ELZONRIS. Interrupt ELZONRIS infusion and provide supportive care as needed if a hypersensitivity reaction should occur.
Hepatotoxicity
- Treatment with ELZONRIS was associated with elevations in liver enzymes. In patients receiving ELZONRIS in clinical trials, elevations in ALT occurred in 79% (96/122) and elevations in AST occurred in 76% (93/122). Grade 3 ALT elevations were reported in 26% (32/122) of patients. Grade 3 AST elevations were reported in 30% (36/122) and Grade 4 AST elevations were reported in 3% (4/122) of patients. Elevated liver enzymes occurred in the majority of patients in Cycle 1 and were reversible following dose interruption.
- Monitor alanine aminotransferase (ALT) and aspartate aminotransferase (AST) prior to each infusion with ELZONRIS. Withhold ELZONRIS temporarily if the transaminases rise to greater than 5 times the upper limit of normal and resume treatment upon normalization or when resolved.
ADVERSE REACTIONS:
Most common adverse reactions (incidence
Please see Full Prescribing Information, including Boxed WARNING.
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This website is intended for
US healthcare professionals only.
Yes, I am a US healthcare professional
You are now leaving ELZONRIS.com/hcp website. Stemline Therapeutics, Inc. does not review the information contained on the following website for content, accuracy, or completeness. Use of and access to the information is subject to the terms, limitations, and conditions set by the website producer. Stemline Therapeutics, Inc. makes no claims about the accuracy or any other aspect of the information contained on this website, nor does Stemline Therapeutics, Inc. endorse the following website.
Add the dosing cycle number and the date of the first dose of the specific dosing cycle to generate a list of dates for the treatment period of the cycle. You can tap/click on ‘Add cycle’ at the bottom to see the dates for future treatment cycles.
Calculate the required dose based on the recommended dosage of ELZONRIS1*:
Patient’s weight
Calculated Dose: 12 mcg/kg X patient’s body weight (kg) =
*Ensure that the required components are available to support the dilution of a second vial, if the calculated dose based on the patient's weight exceeds 10 mL of ELZONRIS.1



